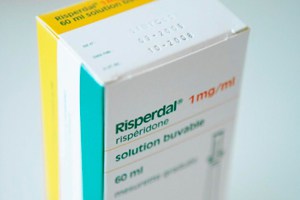
Philadelphia Jury Awards Family $2.5M in Risperdal Lawsuit
A jury in Philadelphia has returned a verdict in a lawsuit against Janssen Pharmaceuticals Inc., parent company Johnson and Johnson for failing to warn parents and doctors of an autistic boy about the dangers of the antipsychotic drug Risperdal. The jury awarded $2.5 million to a family because of the company’s failure to warn the>> Read More
